Vitamin D Analogues
Drug Name |
Molecular Formula |
Background |
Structure |
PubchemLink |
|---|---|---|---|---|
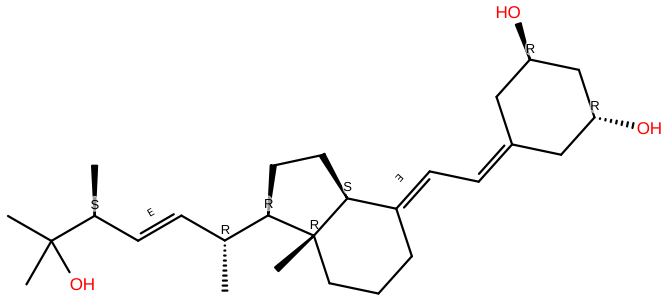
Calcitriol |
C22H44O3 |
Calcitriol is an authorized medicine for the treatment of sHPT in CKD patients in stages 3-5 and for the treatment of metabolic bone disease in dialysis patients. Calcitriol treatment has a significant impact on the correction of vitamin D insufficiency in regular dialysis patients. |
 |
|
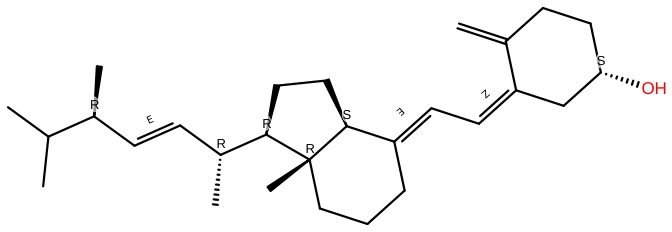
Ergocalciferol |
C28H44O |
Ergocalciferol is used to treat vitamin D insufficiency and increased PTH levels in CKD patients (stages 3-4). The Food and Drug Administration (FDA) has authorized it for the treatment of vitamin D insufficiency. It's a prohormone that the liver and kidney should convert to physiologically active forms of vitamin D |
 |
5280793 |
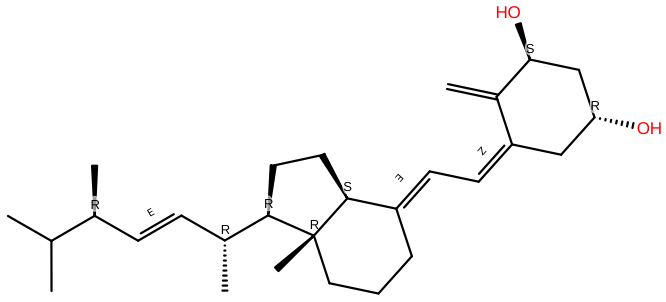
Doxercalciferol |
C28H44O2 |
Doxercalciferol seems to be a second-generation analog that needs to be activated biologically in the liver. It's a medication that's approved for CKD patients between stages 3-5, including those on dialysis. |
 |
5281107 |
Paricalcitol |
C27H44O3 |
Paricalcitol is a vitamin D analog of the third generation. When it binds to VDR, it mimics the activity of calcitriol. It is indicated as an oral form for individuals with CKD stages 3 and 4, as well as those who are not on dialysis. |
 |
5281104 |
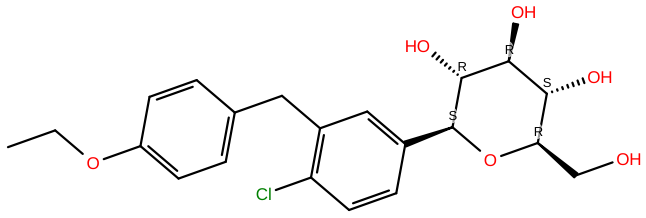
Dapagliflozin |
C21H25ClO6 |
FDA recently approved Farxiga to treat renal disease and kidney failure which is taken by orally. The dialysis and hypersensitivity reactions patients should avoid Farxiga. The side effects of Farxiga are urinary tract infection, dehydration, metabolic acidosis or ketoacidosis, and vaginal yeast infections |
 |
9887712 |